Preparation of Silicade
Silicade as a Synthetic OSA Rich Silica Water Supplement
Making silicon rich water weekly at home is easy and much less expensive and more sustainable than purchasing water bottled in Fiji or Malaysia. I call this water “Silicade”.
The written recipe is below this video.
Silicade provides 124ppm of dissolved silica to lower your body-burden of aluminum. Silicade preparation requires only two ingredients and a set of small measuring spoons that in the U.S.A. can be purchased online and shipped to your home. Silicade can be stored indefinitely in the dark like Fiji water.
The chemicals to make Silicade store well and should be kept out of children’s reach:·
Low Alkalinity Hydrous Sodium Silicate: a hydrous powder available online from ChemicalStore.com. The powder is safer and easier to measure than the liquid form but has the same ratio of 3.22 SiO2 to Na2O. The powder has a as a purity of 99.5% and a formula of SiO2[Na2O]1/3.22 H2O (18.5% water) Mw of 97.25. Only order “sodium silicate – low alkalinity”. Do not order “sodium silicate – alkaline” from the ChemicalStore.com. This powdery chemical can be stored indefinitely in its screw-cap plastic container but slowly clumps. The clumps are easily converted back to powder with a small mortar and pestle.
Note: This solid sodium silicate from the Chemical Store is Product G manufactured by the PQ Corporation of Valley Forge, PA. Brenntag Specialties (Telephone No. 888-926-4151) buys Product G from PQ Corporation and resells it worldwide as G Sodium Silicate product number 387721 in 50 pound bags. ChemicalStore.com and Zchemicals.com buy this product from Brenntag Specialties and sell it in 2 pound containers online.
https://shop.chemicalstore.com/sodium-silicate-low-alkaline?keyword=sodium%20silicate%20&category_id=0
Sodium Bisulfate (a.k.a. Sodium Hydrogen Sulfate): a white powder 99.5% pure of micro-prills (i.e. very small pellets) from Professor Fullwood of LoudWolf Ltd. is available from Amazon. Note: both optional calcium chloride and magnesium chloride are available from the same source.·
Mini Measuring Spoon Set: dash, smidgen, pinch – Mini Measuring Spoons Set Heavy Duty Stainless Steel Measuring Spoons for Dry or Liquid Ingredients
Here is a link to one option for measuring spoons. https://www.amazon.com/Measuring-Stainless-Tablespoon-Teaspoon-Ingredients/dp/B091SW8BD5/ref=ast_sto_dp_puis
Here is a link to a write up for other options for measuring spoons. There are a few choices. https://prevent-alzheimers-autism-stroke.blogspot.com/2023/05/silicade-measuring-spoons-alert-change.html
Do not use antique dash and smidgen measuring spoons as they may not be correctly calibrated. ·
Spatula: Any small spatula with a straight-edge works to level the contents of the measuring spoons prior to addition.
Detailed Instructions with Options for Making Silicade
By following these detailed instructions you can prepare a gallon of Silicade or just follow the “Short Recipe for Silicade” that follows after these detailed instructions:1)
1) A level dash and two level smidgens (3/16 of a teaspoon, 600mg) of hydrous powdered sodium silicate is placed in a Pyrex glass measuring cup. Add 1/8 cup of tap water and bring to boiling in the microwave or on the stove, and let boil for 30sec. This powder contains 99.5% water soluble sodium silicate monohydrate and a maximum of 0.5% of water insoluble materials, as required by the American Waterworks Standard B104-98 for adding sodium silicate to drinking water23.
Note: Do not heat to boiling more than 1/8 cup of tap water as more water will lower the pH making the sodium silicate less soluble.
2) The hot water with dissolved sodium silicate is immediately diluted to one gallon (3.785 liters) with cold tap water resulting in a 1.29 mM/liter (124ppm) solution of pH 9.8 OSA.
3) One level dash (1/8 of a teaspoon, 0.83 gr, 6.9 mM) of sodium bisulfate is added to the solution of OSA and dissolved with stirring in order to acidify the solution to pH 4 to 5. Optionally, if tap water is more basic than pH 8.5, use a pH meter while slowly adding a little more sodium bisulfate in order to lower the pH to 4.0-5.0. A pH 7.0 standard solution is recommended for periodic calibration of the pH meter.
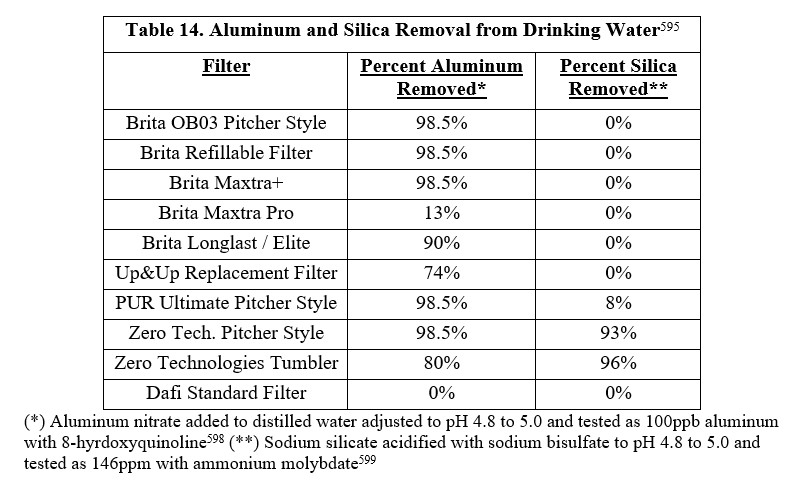
4) The clear colorless acidic solution of OSA is further purified by filtering through a Brita pitcher style filter resulting in OSA at a pH of 4.4. This removes impurities added with sodium silicate and sodium bisulfate. You can use the Standard/original Brita filter, the refillable Brita filter and the Brita Maxtra+ to make Silicade.
5) Two level smidgens of sodium bicarbonate (a.k.a. baking soda) are added and dissolved with stirring in the gallon of filtered OSA, resulting in Silicade with a pH of 6.5, a TDS of 285 at 25oC, and less than 2mcg/L labile aluminum. Each quart of Silicade contains 36.5mg of dissolved silicon as 124ppm of monomeric (OSA).
6) Optionally make Silicade Plus Calcium, if tap water is low in calcium, add two level dashes of calcium chloride flakes or prills (840mg 36% calcium) 99% pure from Loudwolf/Amazon. This will increase the calcium level by 80 ppm, the TDS to 450 at 25oC, and the pH to 6.6 in a gallon of Silicade + Ca. Labile aluminum in calcium enriched Silicade is less than 2mcg/L. Calcium at concentrations greater than or equal to 75ppm have a significant protective effect on cognition433.
Optionally in order to increase magnesium by 20ppm add a dash of magnesium chloride hexahydrate (>98% purity) from LoudWolf/Amazon. Optionally make Sparkling Silicade – Carbonating Silicade will result in a pH 4.5 sparkling beverage.
Drink 3 to 4 cups of Silicade a day around meal times in order to provide a total of 25.5 to 34mg of silicon as monomeric OSA. This is 7.7 to 10.3 times the 3.3mg of silicon that when consumed as OSA per day was observed to lower the frequency of AD118.
Silicade contains 124ppm of OSA and in the U.S.A. 160ppm of OSA (i.e. 100ppm of SiO2) is generally recognized as safe in drinking water22.
Short Recipe for Silicade
Ingredients needed:
Sodium Silicate·
Sodium Bisulfate·
Baking Soda (sodium bicarbonate)
Tools needed:
Dash measuring spoon = 1/8 tsp·
Smidgen measuring spoon = 1/32 tsp·
1 cup Pyrex measuring cup·
1 gallon measuring container·
Brita filter – pitcher style·
Spatula for leveling·
Stirring utensil
Steps:
1. Add 1 level dash & 2 level smidgens of sodium silicate to a one-cup Pyrex container
2. Add 1/8 cup of tap water to the one-cup Pyrex measuring container
3. Heat the contents of the Pyrex measuring cup to boiling and boil for at least 30 seconds
4. Dilute immediately with a small amount of unheated tap water
5. Pour all the contents of the Pyrex measuring cup into a 1 gallon container
6. Fill the 1 gallon container with unheated tap water to the 1 gallon mark on the container
7. Add 1 level dash of sodium bisulfate to the one gallon container
8. Stir the mixture thoroughly and then filter the mixture through a Brita filter pitcher
9. After filtering, add 2 level smidgens of baking soda (sodium bicarbonate) to the mixture
10. Stir Silicade to dissolve the baking soda
11. Enjoy the health benefits of drinking Silicade!
Silicade can be stored indefinitely in the dark at room temperature or in a refrigerator.
Why This Recipe WorksThe goal of this recipe for orthosilicic acid (OSA) in drinking water is to use an easily measured solid silica powder and an acidic microprill that are commercially available online and shipped to anyone, not just chemical laboratories. Both of these chemicals are high purity (e.g. 99.5%). ·
Solubilize sodium silicate: Boiling powdered sodium silicate for 30 seconds in an eighth of a cup of tap water keeps the pH high enough (e.g. pH = 13) to solubilize silicate434-436.·
Neutralize to form OSA and prevent polymerization: In order to form OSA and other silica species in equilibrium with OSA489 and to prevent OSA polymerization435-437, immediately dilute the basic (e.g. pH=13) OSA solution to a gallon with tap water and then immediately render the solution non-hazardous by acidifying the solution to pH 4 to 5 with the solid acid sodium bisulfate. A 1.29mM OSA solution is well below OSA’s saturation level in water (e.g. 2-3mM) but requires 7 days to fully stabilize rising from 108ppm immediately after preparation to 124ppm174. Polymerization of OSA has been observed at neutral pH only well above OSA’s 200ppm saturation level435-437.·
Remove Aluminum: For optimal aluminum removal acidify the OSA solution with sodium bisulfate to pH 4.0 to 5.0 and then filter through a Brita pitcher style filter (OB03)174. A significant portion (e.g. 98.5%) of the labile aluminum introduced in tap water is removed174,175. This Brita filter is a combined activated carbon and weak cation exchange resin that removes cations like aluminum but does not remove OSA174. If the tap water used for Silicade is between pH 6.5 to 8.5, as per EPA’s secondary drinking water standard, then after acidification, filtration, and bicarbonate addition Silicade will be pH 6.5. ·
Optionally add Calcium and/or Magnesium: Have your tap water checked and if it is low in calcium and/or magnesium, add supplemental calcium and/or magnesium to Silicade. The Brita filter reduces calcium and magnesium in Quabbin tap water by one half175. Drinking water with calcium at levels of 80mg and magnesium at levels of 20 ppm has been found to be optimal for good health438. This may be due to calcium and magnesium competing with aluminum for absorption by the gut433. Calcium catalyzes the polymerization of OSA but only at pH greater than 818,19. Silicade + Ca is pH 6.6and at this pH OSA in Silicade + Ca is primarily a non-polymeric monomer174,439.
Answers to frequently asked questions about making Silicade.