Dangers of Alzheimer’s drugs that target Beta-Amyloid
Rung 24 – Counterfactual: Removing Beta-Amyloid is a Cure for AD
Written by Dennis N Crouse PhD
Excerpt from book “Finding a Cause and Potential Cures for Alzheimer’s Disease: Climbing the Ladder of AD Causation” available on Amazon
Slowly accumulating fibrillary B-amyloid (a.k.a. AB, amyloid-B, beta-amyloid plaque, senile plaque), made from a family of peptides (e.g., AB-42 and AB-40) all derived from a common precursor protein (e.g., APP) in the brain, is detected in both AD and non-AD dementia. One approach to finding a cure for AD is based upon the unproven Amyloid Hypothesis of AD that posits: abnormally high levels of B-amyloid accumulation in the brain are the cause of AD437,438.
If the Amyloid Hypothesis is true, removing beta-amyloid should cure AD. However, seventeen Phase III trials, involving 12,585 AD patients, have been unsuccessful in curing AD with monoclonal antibodies that are proven to remove B-amyloid plaque and/or AB-42 oligomers439.
AB-42 oligomers are mediators of aluminum causing AD. Inhibiting their formation with homotaurine (a.k.a. tramiprosate) does stabilize cognition and slow hippocampal atrophy as described in rung 6. However, removing AB-42 oligomers and/or plaque from the brains of AD patients has been shown to be dangerous and result in no significant cognitive improvement .
The Amyloid Hypothesis is counterfactual because B-amyloid plaque is not a cause of AD as it is not present in some AD patients and not specific to AD.440,441. B-amyloid plaque occurs in 88% of people with AD but also in 51% of those with Lewy Body dementia and 30% of those with vascular dementia440.
Because aluminum is the primary cause of AD, removing B-amyloid does not cure AD. The oral ingestion of aluminum ions causes increased of expression APP77. Aluminum ions cause both an increased expression of APP and a 5-fold increase of AB-42 in a human neuroblast cell line51,52,369,471. AB-42 oligomers are 10-fold more neurotoxic than AB-42 fibrils442. Aluminum binds to AB-42 oligomers making them 50% more neurotoxic than aluminum-free AB-42 oligomers53. Therefore, oral aluminum ingestion increases: APP expression, AB-42 levels, amount of neurotoxic aluminum bound AB-42 oligomers, and risk of AD. Avoiding aluminum ingestion and drinking silica rich water to facilitate the elimination of aluminum in urine and perspiration has been shown to improve cognition in some AD patients.
Side Effects of Monoclonal Antibody Treatment
A 2021 meta-analysis of seventeen Phase III randomized controlled trials of anti-AB monoclonal antibodies, involving 12,585 AD patients found that AB mobilization achieved by immunotherapies is causally linked by dose response to both increased B-amyloid removal and increased life-threatening risk of edema and hemorrhaging (i.e., amyloid-related imaging abnormalities ARIA-E and ARIA-H respectively)439,444. Therefore, removing B-amyloid from the brain with monoclonal antibody treatment should improve AD symptomology according to the “Amyloid Hypothesis” but instead increases the risk of ARIA-E and ARIA-H. This can be described as the “ARIA Paradox” 444.
AB mobilization achieved by some monoclonal antibody treatments appears to be causally linked by dose response to both increased B-amyloid removal and increased rate of brain atrophy. This can be described as the “Brain Atrophy Paradox” because removing B-amyloid from the brain with monoclonal antibody treatment should improve AD symptomology according to the “Amyloid Hypothesis” but instead increases the rate of brain atrophy a biomarker of AD.
Slow brain atrophy is common in the elderly, but accelerated brain atrophy is both a biomarker of AD and a mediator in aluminum causing AD (rung 4). It is known that aluminum causes neurodegeneration and neuron loss84. Accelerated brain atrophy in AD is due to neuron loss143. Also, brain atrophy is associated with the progression of AD144. Aluminum accumulates in the same regions of the brain where accelerated brain atrophy is observed in AD23,31.
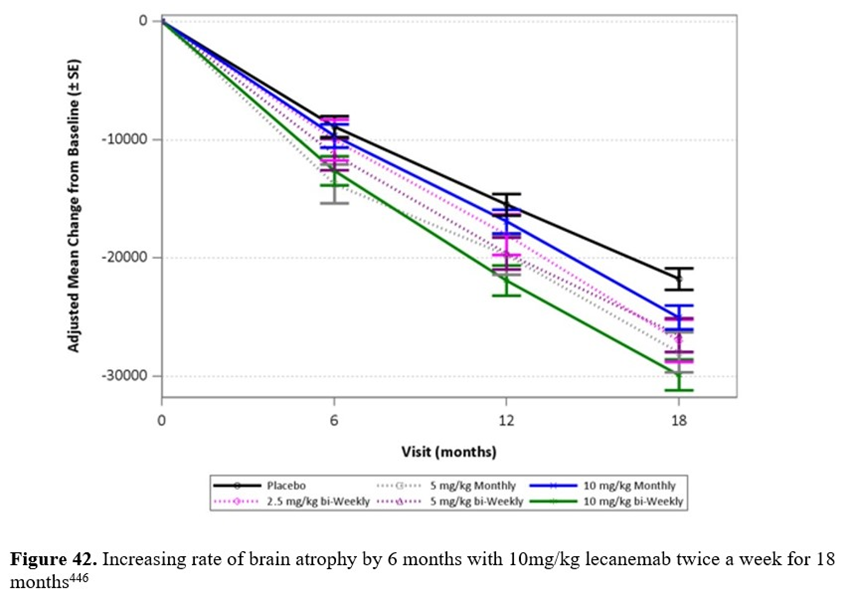
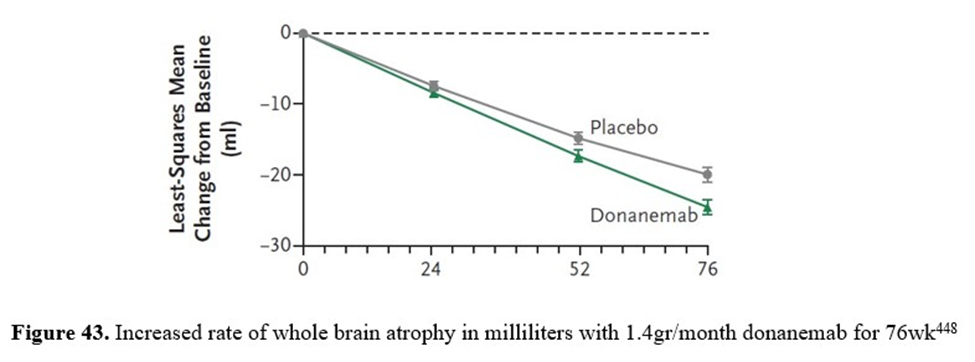
Brain atrophy is measured on MRI scans of the brain. Brain atrophy is observed by volumetric MRI as decreasing whole-brain volume and/or increasing volume of the brain’s fluid-filled spaces (a.k.a. ventricular volume). The data in figures 42 and 43, indicating accelerated brain atrophy in AD patients treated with two anti-AB monoclonal antibodies (i.e., lecanemab and donanemab), is supporting the contention that removing B-amyloid is not a cure but is worsening degenerative changes in the brains of people with AD. These changes may result in long-term worsening of cognitive decline445.
Examples of Anti-AB Monoclonal Antibody (AAMA) Treatments
Lecanemab (a.k.a. Leqembi) is an anti-AB monoclonal antibody that binds to AB-oligomers and AB-protofibrils. In 2023 based upon the observation that AB-plaques are reduced by lecanemab treatment, the U.S. FDA has given the drug an accelerated approval contingent upon verification of clinical benefit. A 2021 study of treatment with lecanemab found accelerated brain atrophy in the group treated with lecanemab versus the placebo group446. The dose dependent increase in the rate of brain atrophy is plotted in figure 42 versus the placebo group indicating that lecanemab causes accelerated brain atrophy accelerating the progression of AD by 6 months compared with the placebo group during the 18-month test.
Participants of the lecanemab study had early symptomatic AD (i.e., MCI) and had AB-plaque. Seventy percent of the participants were ApoE E4 carriers (homozygous and heterozygous) and those carriers on the highest dose (10mg/kg twice a week) had the highest risk of developing symptomatic and life-threatening ARIA possibly because of more AB-oligomers446. Ten percent of all participants taking the highest dose of lecanemab developed ARIA446.
After treatment at the high dose of 10mg/kg of lecanemab twice a week there was no significant improvement in cognition, as tested by Clinical Dementia Rating Sum-of-Boxes (CDR-SB) and Alzheimer’s Disease Composite Score (ADCOMS)447. However, there was a slower loss of cognition in the treated group during the first 10 months of treatment as opposed to the placebo group. After the first 9 months the rate of cognitive decline in the treated group resumed and remained equal to the placebo group even after a gap in treatment, indicating no clinical benefit in long term cognitive decline447. AB-oligomers and AB-protofibrils were significantly reduced only during the first 6 months of treatment447.
With no significant improvement in cognition in those treated with high dose lecanemab, there appears to be insufficient clinical benefit in removing AB-oligomers and AB-protofibrils. In fact, the accelerated brain atrophy caused by lecanemab only speeds the progression of AD. This data calls into question the validity of the Amyloid Hypothesis as removal of AB-oligomers and AB-protofibrils did not significantly improve cognition but increased brain atrophy.
Donanemab is an IgG1 antibody targeting an N-terminal pyroglutamate AB epitope that is present in only established AB-plaque448. In 2021 a study of treatment for 76 weeks with donanemab found accelerated brain atrophy due to decreased whole-brain volume and increased ventricular volume versus the placebo group448. The difference in brain volume between placebo and donanemab treatment is 5 cubic centimeters (i.e., 5cc). This volume of accelerated brain atrophy has accelerated the progression of AD by 15 weeks in those treated with donanemab for 76 weeks compared with the placebo group (see figure 43).
Participants for the donanemab study were diagnosed with early symptomatic AD and had AB-plaque. Eighty percent of the total amount of B-amyloid removed was removed after 24 weeks of treatment and 60% of treated participants had B-amyloid-negative status by 52 weeks. In addition, after 76 weeks the donanemab treated patients had no cognitive improvement but decline was smaller compared with those given a placebo, as measured by the Integrated Alzheimer’s Disease Rating Scale (iADRS), that is a composite score of cognition (ADS-Cog) and daily living skills (ADCS-iADL). However, 25% of participants had ARIA-E with 6% having symptomatic ARIA-E448.
Bapineuzumab is a an IgG1 antibody targeting five N-terminal residues on AB and can clear both AB-oligomers and AB-protofibrils. In 2016 it was reported that after 71 weeks bapineuzumab-treated patients had significantly accelerated brain atrophy as indicated by an increase in ventricular volume versus the placebo-treated group449. Bapineuzumab decreased brain volume by approximately 2 milliliters compared to the placebo after 78 weeks of treatment450. ARIA-E occurred in 15.3% of ApoE E4 carriers who received bapineuzumab treatment (0.5mg/kg).
Aducanumab (a.k.a. Aduhelm) is a human monoclonal antibody that targets and clears both AB-oligomers and AB-protofibrils, as does Bapinezumab451. In 2022 results of low-dose and high-dose treatment with aducanumab for 78 weeks in both the identical EMERGE and ENGAGE studies found significant accelerated brain atrophy as an increase in ventricular volume of <0.2% (p<0.0001) versus the placebo-treated group452. Both the identical EMERGE and ENGAGE studies were terminated on March 21 2019 due to insignificant clinical improvement of participants and the high frequency of ARIA-E. The frequency of ARIA-E in high dose group of the ENGAGE study was 36% and in the EMERGE study it was 35%452.
However, by ignoring the results of the ENGAGE study and looking only at the participants who received high-dose treatment in the EMERGE study, there was no cognitive improvement but cognitive decline was smaller with aducanumab treatment versus the placebo group.
Biogen in June 2021 was granted accelerated approval by the U.S. FDA of aducanumab for anti-AB-treatment for AD, in spite of dissenting views from the FDA’s own statistical team who said there was inadequate evidence that aducanumab actually clinically benefited AD patients. The accelerated approval is controversial because it is based upon an unproven assumption that removal of B-amyloid is a reasonable surrogate for unobserved clinical benefit.
In April 2022 the Centers for Medicare and Medicaid Services (CMS) issued an unprecedented decision that all but denies coverage of FDA-approved treatments for AD that target B-amyloid. Also, major health systems, including the Cleveland Clinic and the Department of Veterans Affairs, decided not to offer aducanumab treatment, citing its uncertain benefits and risks of brain swelling/edema (ARIA-E) and brain bleeding/ hemorrhaging (ARIA-H). Biogen’s annual price of aducanumab, currently $28,800, and lack of health care support essentially sidelined aducanumab (a.k.a. Aduhelm) from the marketplace.
Anti-AB Monoclonal Antibody Therapy is Accelerating Brain Atrophy
The observed volume of brain atrophy caused by anti-AB monoclonal antibody therapy is approximately 1,000 times larger than the volume of removed B-amyloid. In addition, accelerated brain atrophy begins after removal of B-amyloid:
Accelerated brain atrophy due to anti-AB monoclonal antibody therapy is as much as 1,000 times greater in volume than total B-amyloid volume loss448
Estimates of total B-amyloid-load in the Alzheimer’s brain is approximately 6.5mg453. Since the density of protein is 1.35g/cc, the total B-amyloid volume is 0.0048cc [i.e., volume in cc = (6.5mg/1350mg/cc)]. In the case of donanemab, the difference in brain volume between placebo and donanemab-treated AD patients was approximately 5cc450. This 5cc is approximately a thousand times larger than estimates of total B-amyloid load (i.e., 0.0048cc) in the Alzheimer’s brain450. Therefore, observed brain atrophy due to anti-AB monoclonal antibody therapy is not due to reduction in B-amyloid-load.
- Eighty percent of total B-amyloid volume loss occurs in the first 24 weeks of anti-AB monoclonal antibody therapy, while the start of accelerated brain atrophy is delayed to 24 weeks after starting anti-AB monoclonal antibody therapy448.
In the donanemab trial, 80% of the total B-amyloid reduction (i.e., 67.83 out of 85.06 centiloids more than the placebo group as assessed by 18F-florbetapir PET) occurred in the first 24 weeks of the trail, while 99% of accelerated brain atrophy was delayed to after 24 weeks (see figure 43)448,454. This temporality supports the contention that donanemab causes accelerated brain atrophy after causing B-amyloid reduction.
Death Due to AAMA
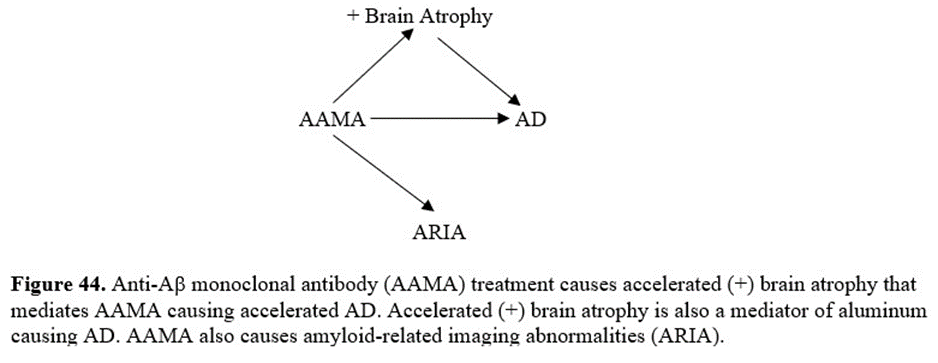
Anti-AB monoclonal antibodies (AAMA) also cause both ARIA-E and ARIA-H during removal of B-amyloid as diagrammed in figure 44 and can result in death455. For instance, across the two aducanumab studies (EMERGE and ENGAGE) there were 5 deaths in the placebo group and 11 deaths in the anti-AB monoclonal antibody therapy group452.
In the Clarity AD-study a 65-year-old woman receiving lecanemab treatment had both ARIA-E and ARIA-H. She died of massive hemorrhaging after being given tissue plasminogen activator (tPA) at Northwestern University Medical Center in Chicago455. Upon autopsy it was found she had a comorbidity: amyloid deposits surrounding some of her brain’s blood vessels [i.e., cerebral amyloid angiopathy (CAA)]455. Because the prevalence of CAA is estimated to be 48% among those diagnosed with AD and 5% to 7% in cognitively normal elderly, this case should be a warning to anyone either thinking of using lecanemab therapy or using tPA or a blood thinner during lecanemab therapy456.
Conclusion of the 24th Rung
After seventeen unsuccessful Phase III randomized controlled trials of anti-AB monoclonal antibodies (AAMA), involving 12,585 AD patients439, the Amyloid Hypothesis has been proven to be counterfactual. Removing B-amyloid does not improve cognition nor is it a cure for AD. Instead, B-amyloid removal causes an increase in the rate of brain atrophy and the occurrence of amyloid-related imaging abnormalities (ARIA). At best, in one of two identical studies (i.e., EMERGE not ENGAGE) removing B-amyloid with aducanumab only slowed the progression of cognitive decline but increased both ARIA and accelerated brain atrophy and neither cured AD nor improved cognition. Also, in the Clarity AD study removing B-amyloid with lecaneamab only slightly slowed the progression of cognitive decline by 10 months but increased ARIA, accelerated brain atrophy by 6 months and neither cured AD nor improved cognition.
Removing B-amyloid is not a cure for AD. Instead, from rungs 1 to 24 it is clear that aluminum accumulation is the cause of MCI and AD and facilitating the elimination of aluminum with orthosilicic acid rich drinking water can improve cognition, prevent MCI and AD, and possibly cure MCI and AD.
Disclaimer – Monoclonal antibody treatment targeted to proteins on specific viral-variants in order to achieve viral immunity is safe and efficacious having saved millions of lives during the Covid-19 pandemic. Anti-AB monoclonal antibody (AAMA) therapy targeted to specific peptides and protofibrils has also been shown to lower levels of B-amyloid in the body. However, AAMA therapy to lower levels of B-amyloid in the body has not been shown to have sufficient clinical benefit to offset its risk of ARIA and accelerated brain atrophy. Therefore, the author supports the use of monoclonal antibody treatment for viral immunity but not for anti-AB therapy.
- Hardy J. and Selkoe, D.J.; The amyloid hypothesis of Alzheimer’s doisease: progress and problems on the road to therapeutics; Science; 297:353-356 PubMed: 12130773
- Selkoe, D.J., and Hardy, J.; The amyloid hypothesis of Alzheimer’s disease at 25 years; EMBO Mol. Med.; 8:595-608 (2016)
- Avgerinos, K.I., et al.; Effects of monoclonal antibodies against amyloid-B on clinical and biomarker outcomes and adverse event risks: a systematic review and meta-analysis of phase III RCTs in Alzheimer’s disease; Ageing Res, Rev.; July; 68:101339. Doi:10.1016/j.arr.2021.11339 (2021)
- Ossenkoppele, R., et al.; Prevalence of PET positivity in dementia syndromes – A meta-analysis; JAMA; 313(19):1939-49 (2015)
- Mohsen, K., et al.; 18F-FDG is a superior indicator of cognitive performance compared to 18F-florbetapir in Alzheimer’s disease and mild cognitive impairment evaluation: a global quantitative analysis; J. Alzheimer’s Dis.; 70(4):1197-1207 (2019)
- Dahlgren, K.N., et al.; Oligomeric and fibrillary species of amyloid-B peptides differentially affect neuronal viability; J. Biol. Chem.; Aug., 277(35):3206-53 (2002)
- Zhang, Q., et al.; Effects of aluminum on amyloid-beta aggregation in the context of Alzheimer’s disease; Arabian J. Chem.; 12:2897-2904 (2019)
- DiFrancesco, J.C., et al.; Anti-Abeta autoantibodies in amyloid related imaging abnormalities (ARIA): Candidate biomarker for immunotherapy in Alzheimer’s disease and cerebral amyloid angiopathy; Frontiers in Neurol.; 6, 207 PubMed: 26441825
- Thambisetty, M.; Anti-amyloid drugs and the mystery of treatment associated brain shrinkage; https://www.statnews.com/2022/11/28/anti-amyloid-drugs-treatment-associated-brain-shrinkage/ (2022)
- Swanson, C.J; et al.; A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer’s disease with lecanemab, an anti-AB protofibril antibody; Alzheimers Res. Ther.; Supplemental figure S4B; Apr., 13(1):80 (2021)
- McDade, E., et al.; Lecanemab in patients with early Alzheimer’s disease: detailed results on biomarker, cognitive, and clinical effects from the randomized and open-label extension of the phase 2 proof-of-concept study; Alzheimer’s Res. Ther.; 14:191 (2022)
- Mintun, M.A., et al.; Donanemab in early Alzheimer’s disease; N. Engl. J. Med.; May, 384(18):1691-1704 (2021)
- Novak, G., et al.; Changes in brain volume with Bapineuzumab in mild to moderate Alzheimer’s disease; J. Alzheimer’s Dis.; 49:1123-34 (2016)
- Ayton, S.; Bain volume loss due to donanemab; Eur. J. Neurol.; June; 28:e67-e68 (2021)
- Sevigny, J., et al.; The antibody aducanumab reduces AB plaques in Alzheimer’s disease; Nature; 537:50-6 (2016)
- Haeberlein, S.B., et al.; Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease; J. Prev. Alz. Dis.; March; http://dx.doi.org/10.14283/jpad.2022.30 (2022)
- Roberts, B.R., et al.; Biochemically defined pools of amyloid-beta in sporadic Alzheimer’s disease: Correlation with amyloid PET; Brain; 140:1486-98 (2017)
- Clark, C.M., et al.; Use of Florbetapir-PET for imaging B-amyloid pathology; JAMA; Jan.; 305(3):275-83 (2011)
- Pillar, C.; Second death linked to potential antibody treatment for Alzheimer’s disease; https://www.science.org/content/article/second-death-linked-potential-antibody-treatment-alzheimer-s-disease ; Science; Nov. (2022)
- Jakel, L., et al.; Prevalence of cerebral amyloid angiopathy: A systematic review and meta-analysis; Alzheimer’s & Dementia; 18:10-28 (2022)